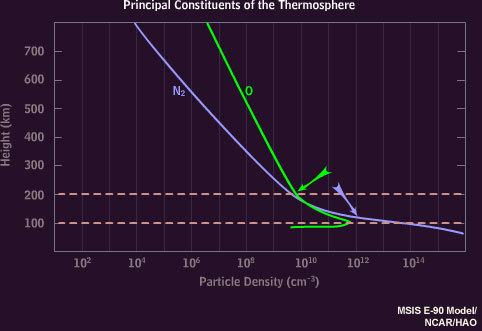
The color of the Aurora depends on the altitude and the atom being struck by solar radiation (causing excitation). At higher altitudes, there is more Atomic Oxygen than Nitrogen, leading to the common color stratifications you see.
500-200 km altitude
— Atomic Oxygen — Red
200-100 km
— Atomic Oxygen — Greenish-Yellow
— Ionized Nitrogen — Blue/Purple
100-80 km
— Nitrogen (N2) — Crimson
Oxygen only emits red at higher altitudes because once it’s excited, it takes a longer time to emit red than it does green. Why is that important? Well, at lower altitudes there is more Nitrogen for the Oxygen to bump into and absorb that excitation-energy before it gets a chance to emit red light. In this case, where the collision occurs, the Oxygen will emit Green and at low enough altitudes the Nitrogen-Oxygen collisions eventually prevent Oxygen from emitting any light at all.
During stronger storms, high energy solar particles will reach lower in the atmosphere and cause the Crimson emission from Nitrogen, creating a deep-red band at the lower edge of the aurora. Other elements emit light too, like Hydrogen (Blue) or Helium (Purple) which are at higher altitudes. When was the last time you saw The Aurora Borealis? If you live in an urban area, chances are, you have never seen it because of light pollution and atmospheric haze. There was an aurora incident here in Europe last night and a cloudless sky. I don't have a light pollution problem. It was pretty cool!500-200 km altitude
— Atomic Oxygen — Red
200-100 km
— Atomic Oxygen — Greenish-Yellow
— Ionized Nitrogen — Blue/Purple
100-80 km
— Nitrogen (N2) — Crimson
Oxygen only emits red at higher altitudes because once it’s excited, it takes a longer time to emit red than it does green. Why is that important? Well, at lower altitudes there is more Nitrogen for the Oxygen to bump into and absorb that excitation-energy before it gets a chance to emit red light. In this case, where the collision occurs, the Oxygen will emit Green and at low enough altitudes the Nitrogen-Oxygen collisions eventually prevent Oxygen from emitting any light at all.

No comments:
Post a Comment